
91 22 2674 3405
91 22 2674 3405

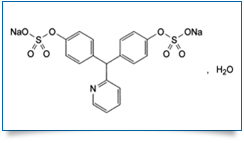 Bulk Active : Sodium Picosulfate
Bulk Active : Sodium Picosulfate
MOLECULAR FORMULA : C18H13NNa2O8S2,H2O
MOLECULAR WEIGHT : 499.4
Pharmacologic Activity :
Laxative
CAS No : 10040-45-6
Specifications : BP/EP
Regulatory compliance : eDMF(CTD)
| TESTS | EP 7.4/BP 2013 |
| Appearance | White or almost white, crystalline powder |
| Solubility | Freely soluble in water, slightly soluble in ethanol (96%). |
IDENTIFICATION Test A : IR Test B : T.L.C Test C: Test D: Test E: |
Infrared absorption spectrum of sample should be concordant with the reference spectrum of Sodium Picosulphate reference standard. The principal spot in the chromatogram obtained with test solution (b) is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a). A white precipitate is formed. A violet colour develops. The solution S gives reaction of sodium |
| Appearance of solution |
Solution S is clear and not more intensely colored than reference solution GY7 |
| Acidity or alkalinity | Not more than 0.25 ml of 0.01 M sodium hydroxide Is required to change the colour of the indicator to pink |
| Related substance (HPLC) Impurity A Impurity B Unspecified Impurities Total Impurity |
NMT 0.2 per cent; NMT 0.2 per cent; NMT 0.10 % NMT 0.5 per cent; |
| Chlorides | Maximum 200 ppm. |
| Sulphates | Maximum 400 ppm. |
| Water content | 3.0 % to 5.0%. |
| Assay | Between 98.5% and 100.5% of C18H13NNa2O8S2 calculated on anhyrous basis |