
91 22 2674 3405
91 22 2674 3405

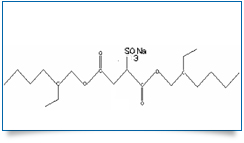 Bulk Active : Docusate Sodium
Bulk Active : Docusate Sodium
MOLECULAR FORMULA : C20H37NaO7S
MOLECULAR WEIGHT : 444.6
Pharmacologic Activity :
Laxative
CAS No : 577-11-7
Specifications : BP/EP/USP
Regulatory compliance : eDMF(CTD)
| TEST | USP 36 | BP 2013/EP 7.4 |
| CHARACTERS | White, wax-like, plastic solid, having a characteristic odor suggestive of octyl alcohol, but no odor of other solvents |
White or almost white, waxy masses or flakes, hygroscopic |
| SOLUBILITY | Very soluble in solvent hexane; freely soluble in alcohol and in glycerin; sparingly soluble in water. | Sparingly soluble in water, freely soluble in ethanol 96% and in methylene chloride. |
IDENTIFICATION TEST A TEST B |
The infrared absorption spectrum should be concordant with USP. reference spectrum of Docusate sodium. ------ |
Infrared absorption spectrum of sample should be concordant with the Ph. Eur reference spectrum of Docusate Sodium Gives reaction for sodium |
| Clarity of solution | Solution (25gm in 100ml alcohol) should not develop a haze within 24 hrs | ------ |
| Alkalinity | ----- | Not more than 0.2 ml of 0.1 M hydrochloric acid is required to change the colour of the indicator to red |
| Related Non-ionic substances | ----- | NMT 0.4 per cent. |
| Limit of bis ( 2-ethylhexyl) maleate | NMT 0.4 %. | ----- |
| Chlorides | ----- | Maximum 350 ppm |
| Water | Not more than 2.0 %. | Maximum 3.0 per cent. |
| Sodium sulphate |
----- |
Maximum 2% |
| RESIDUE ON IGNITION | Between 15.5% and 16.5%, calculated on anhydrous basis | ----- |
| HEAVY METALS | Not more than 10 ppm | Maximum 10 ppm |
| ASSAY | NLT 99.0% and NMT 100.5% of C20H37NaO7S, calculated on the anhydrous basis. | 98.0% to 101.0% per cent of C20H37NaO7S calculated on anhydrous substance. |