
91 22 2674 3405
91 22 2674 3405

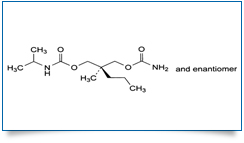 Bulk Active : Carisoprodol
Bulk Active : Carisoprodol
MOLECULAR FORMULA :C12H24N2O4
MOLECULAR WEIGHT : 260.3
Pharmacologic Activity :
Skeletal muscle relaxant
CAS No : 78-44-4
Specifications : BP/USP
Regulatory compliance : Under preparation
| TEST | USP 36 | BP 2013/EP 7.4 |
| CHARACTERS | White crystalline powder having characteristic odor and bitter taste | White or almost white, fine powder |
| SOLUBILITY | Very slightly soluble in water, freely soluble in acetone, in alcohol and in chloroform | Very slightly soluble in water, freely soluble in acetone, in alcohol and in methylene chloride. |
| IDENTIFICATION
MELTING POINT INFRARED ABSORPTION TEST C TEST D |
----- IR spectra concordant with reference sample The retention time of the major peak in the Sample solution corresponds to that in the Standard solution as obtained in the Assay. ------ |
92 °C to 95 °C. Infrared absorption spectrophotometry Comparison carisoprodol CRS. Examine the chromatograms obtained in the test for related substances. The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (d). An intense blue colour develops |
| OPTICAL ROTATION | ----- | - 0.10° to + 0.10°. |
| RELATED SUBSTANCE IMP D Carisoprdol related compound A Meprobamate Carisoprodol monocarbamate Any other unknown individual impurity Total impurities |
----- 0.1 0.5 0.1 0.1 1.0 |
0.5 % ----- ----- ----- 0.2% ----- |
| LOSS ON DRYING | NMT 0.5% | NMT 0.5 % w/w |
| SULPHATED ASH | ----- | NMT 0.10% |
| RESIDUE ON IGNITION | NMT 0.1 per cent, | --------- |
| HEAVY METALS | NMT 10PPM | NMT 10PPM |
| ASSAY | NLT 98.0% and NMT 102.0% of C12H24N2O4 , calculated on the dried basis. | 98.0 per cent to 102.0 per cent (dried substance) |