
91 22 2674 3405
91 22 2674 3405

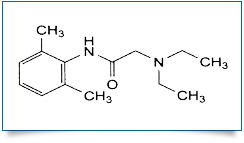 Bulk Active : Lidocaine
Bulk Active : Lidocaine
MOLECULAR FORMULA : C14H22N2O
MOLECULAR WEIGHT : 234.3
Pharmacologic Activity :
Local Anaesthetic
CAS No : 137-58-6
Specifications : BP/EP/USP
Regulatory compliance : eDMF(CTD)
| TEST | USP 36 | BP 2013/EP 7.4 |
| CHARACTERS | White or slightly yellow, crystalline powder. Has a characteristic odor and is stable in air |
White or almost white, crystalline powder |
| SOLUBILITY | Very soluble in alcohol and in chloroform; freely soluble in benzene and in ether ; Practically insoluble in water Dissolves in oils. | practically insoluble in water, very soluble in ethanol 96% and in methylene chloride. |
IDENTIFICATION Test A: I R Test B: Melting point Test C: |
Infrared absorption spectrophotometry to compare with the spectrum obtained with lidocaine CRS. ----- The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay |
Infrared absorption spectrophotometry to compare with the spectrum obtained with lidocaine CRS. 66°C to 70°C. determined without previous drying A green color should be produced. It complies with the water test |
| Related Substance by HPLC impurity A unspecified impurities total impurities |
----- | NMT 0.01 % for each impurity, NMT 0.10 % NMT 0.5 % |
| Chlorides | NMT 35 ppm | NMT 35ppm |
| Sulphates | NMT 0.1% | NNMT 0.1% |
| Water | ----- | NMT 1.0% % w/w |
| Residue on ignition | NMT 0.1% | ----- |
| Heavy metals | Less than 20 ppm | ----- |
| Sulphated Ash |
----- |
NMT 0.1% |
| Assay | Between 97.5% to 102.50% | Between 99.0% to 101.0% calculated on anhydrous basis |