
91 22 2674 3405
91 22 2674 3405

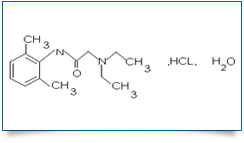 Bulk Active : Lidocaine Hydrochloride
Bulk Active : Lidocaine Hydrochloride
MOLECULAR FORMULA : C14H22N2O,HCL,H2O
MOLECULAR WEIGHT : 288.8
Pharmacologic Activity :
Local Anaesthetic
CAS No : 6108-05-0
Specifications : BP/EP/USP
Regulatory compliance : eDMF(CTD)
| TEST | USP 36 | BP 2013/EP 7.4 |
| DESCRIPTION | White ,odorless, crystalline powder ,having a slightly bitter taste. | White or almost white, crystalline powder |
| SOLUBILITY | Very soluble in water and in alcohol; soluble in chloroform; insoluble in ether. | Very soluble in water, freely soluble in ethanol (96%). |
IDENTIFICATION Test A:Melting point Test B: I. R Test C: Test D: Test E: |
75°C to 77°C ----- Infrared absorption spectrophotometry to compare with spectrum obtained with Lidocaine Hcl reference standard ----- Responds to test for chlorides. The retention time of the major peak of the sample solution corresponds to that of the standard solution as obtained in the Assay solution of Lidocaine Hydrochloride |
74°C to 79°C, determined without previous drying. Infrared absorption spectrophotometry to compare with the spectrum obtained with lidocaine hydrochloride CRS A green color should be produced. Confirms to test for chlorides ---- |
| Appearance of solution | ----- | Solution S is clear and colorless |
| pH | ----- | 4.0-5.5 |
| Related Substance by HPLC impurity A unspecified impurities total impurities |
----- | NMT 0.01 % for each impurity, NMT 0.10 % NMT 0.5 % |
| Heavy metals | NMT 20ppm | Less than 5 ppm |
| Water | 5.5- 7.0 % w/w | 5.5- 7.0 % w/w |
| Sulphated Ash | ----- | NMT 0.1% |
| Residue on ignition |
NMT 0.1% |
----- |
| Sulphates | NMT 0.1% | ----- |
| Assay | Between 97.50% to 102.5% calculated on anhydrous basis |
Between 99.0% to 101.0% calculated on anhydrous basis |